| Identification |
| Name: | Cobalt sulfate |
| Synonyms: | Sulfuric acid,cobalt(2+) salt (1:1); |
| CAS: | 10124-43-3 |
| EINECS: | 233-334-2 |
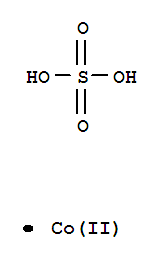
| Molecular Formula: | CoO4S |
| Molecular Weight: | 154.99 |
| InChI: | InChI=1S/Co.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2 |
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 3082 |
| Density: | g/cm3 |
| Stability: | Stable at normal temperatures and pressures. |
| Solubility: | 362 g/L |
| Appearance: | Odorless rose-pink solid. |
| Specification: |
?Cobalt Sulfate (CAS NO.10124-43-3)?is also called Cobalt (2+) sulfate ;C obalt sulfate (1:1) ; Cobalt(ii) sulfate (1:1) .? Acidic salts, such as?Cobalt Sulfate (CAS NO.10124-43-3), are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Inhalation causes shortness of breath and coughing; permanent disability may occur. Ingestion causes pain and vomiting. Contact with eyes or skin causes irritation. Special Hazards of Combustion Products: Toxic cobalt oxide fumes may form in fire.
|
| Storage Temperature: | Cool, dry location. Tightly sealed container. |
| Color: | Red to lavender dimorphic, othorhombic crystals
Red orthorhombic crystals
Red powder |
| Usage: | Used the preparation of pigments, as well as in the manufacture of other cobalt salts. Cobalt pigment is used in porcelains and glass. |
| Safety Data |
| Hazard Symbols |
 T: Toxic
T: Toxic
 N: Dangerous for the environment
N: Dangerous for the environment
|
| |
 |