| Identification |
| Name: | Cobalt oxide (CoO) |
| Synonyms: | C.I. 77322;C.I. Pigment Black 13; Cobalt Black; Cobalt monooxide; Cobalt monoxide; Cobaltoxide; Cobalt(2+) oxide; Cobalt(II) oxide; Cobaltous oxide; Cobaltous oxide(CoO); FCO 178; Zaffre |
| CAS: | 1307-96-6 |
| EINECS: | 215-154-6 |
| Molecular Formula: | CoO |
| Molecular Weight: | 74.93, 165.86, 240.80 |
| InChI: | InChI=1S/Co.O |
| Molecular Structure: |
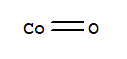 |
| Properties |
| Transport: | UN 3288 |
| Melting Point: | 895 C (Decomposes) |
| Boiling Point: | 3800 C |
| Density: | 6.45 |
| Stability: | Stability Stable, but may be moisture sensitive. |
| Water Solubility: | Insoluble |
| Solubility: | Insoluble |
| Appearance: | Black-gray crystalline powder |
| Specification: |
Cobalt(II) Oxide (CAS NO.1307-96-6) is a olive green, red, gray or black, odorless powder.
First Aid Measures:
Ingestion: Never give anything by mouth to an unconscious person. Get medical aid. Do?not induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Seek medical attention. If individual is drowsy or unconscious, do not give anything by mouth; place individual on the left side with the head down. Contact a physician, medical facility, or poison control center for advice about whether to induce vomiting. If possible, do not leave individual unattended.
Inhalation: Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. If symptoms develop, move individual away from exposure and into fresh air. If symptoms persist, seek medical attention. If breathing is difficult, administer oxygen. Keep person warm and quiet; seek immediate medical attention. May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Skin: May cause skin irritation.Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Remove contaminated clothing and wash exposed area thoroughly with soap and water. A physician should examine the area if irritation or pain persists.
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.May cause eye irritation. Irrigate exposed eyes with copious amounts of tepid water for at least 15 minutes. If irritation, pain, swelling, lacrimation, or photophobia persist, the patient should be seen in a health care facility.
Handling and Storage:
Storage: Kepp container tightly closed. Suitable for any general chemical storage area. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. All chemicals should be considered hazardous. Avoid direct physical contact. Use appropriate, approved safety equipment. Untrained individuals should not handle this chemical or its container. Handling should occur in a chemical fume hood.
|
| Report: |
IARC Cancer Review: Group 2B IMEMDT ?? IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.:?) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT ?? IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.:?) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT ?? IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.:?) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Cobalt and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
|
| Packinggroup: | III |
| Storage Temperature: | Kepp container tightly closed. Suitable for any general chemical storage area. |
| Sensitive: | Air Sensitive |
| Color: | Powder or cubic or hexagonal crystals; color varies from olive green to red depending on particle size, but the commercial material is usually dark gray
Gray cubic crystals
Grayish powder under most conditiond; can form green brown crystals. |
| Usage: | In pigments for ceramics, glass coloring & decolorization, prepn of cobalt metal catalysts, oxidation catalyst for drying oils, fast drying paints & varnishes, cobalt powder for binder in sintered tungsten carbide, in semiconductors. |
| Safety Data |
| Hazard Symbols |
 Xn: Harmful
Xn: Harmful
 N: Dangerous for the environment
N: Dangerous for the environment
|
| |
 |