| Identification |
| Name: | Chloric acid, bariumsalt (2:1) |
| Synonyms: | Bariumchlorate (6CI,7CI); Chloric acid, barium salt (8CI,9CI); Barium chlorate(Ba(ClO3)2) |
| CAS: | 13477-00-4 |
| EINECS: | 236-760-7 |
| Molecular Formula: | Ba. 2 Cl H O3 |
| Molecular Weight: | 304.24 |
| InChI: | InChI=1/Ba.ClHO3/c;2-1(3)4/h;(H,2,3,4)/q+2;/p-1 |
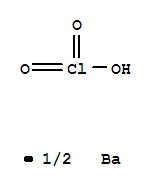
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 1445 5 |
| Flash Point: | °C |
| Boiling Point: | °Cat760mmHg |
| Solubility: | Slightly soluble in ethanol, acetone
37.9 g/ 100 g water at 25 deg C |
| Appearance: | white solid |
| Specification: |
Barium chlorate (CAS No.13477-00-4) is a white crystalline solid. It is an irritant, and if consumed can cause nausea, vomiting, and diarrhoea. It is used in pyrotechnics to produce a green colour.
|
| Report: |
Reported in EPA TSCA Inventory. Barium and its compounds are on the Community Right-To-Know List.
|
| Packinggroup: | II |
| Flash Point: | °C |
| Color: | White crystals |
| Safety Data |
| Hazard Symbols |
 A poison. TLV: see Barium. Strong oxidizer, fire risk in contact with organic materials.
A poison. TLV: see Barium. Strong oxidizer, fire risk in contact with organic materials.
|
| |
 |