| Identification |
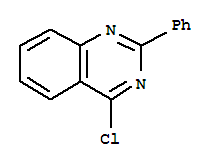
| Name: | Quinazoline,4-chloro-2-phenyl- |
| Synonyms: | 2-Phenyl-4-chloroquinazoline;4-Chloro-2-phenylquinazoline; NSC 400965 |
| CAS: | 6484-25-9 |
| EINECS: | 229-346-2 |
| Molecular Formula: | C14H9 Cl N2 |
| Molecular Weight: | 240.6877 |
| InChI: | InChI=1/C14H9ClN2/c15-13-11-8-4-5-9-12(11)16-14(17-13)10-6-2-1-3-7-10/h1-9H |
| Molecular Structure: |
 |
| Properties |
| Melting Point: | 124-126 °C(lit.) |
| Flash Point: | 164.4°C |
| Boiling Point: | 301.2°Cat760mmHg |
| Density: | 1.285g/cm3 |
| Refractive index: | 1.667 |
| Appearance: | slightly yellow to yellow crystalline powder |
| Specification: |
slightly yellow to yellow crystalline powder
usageEng:Reactnat involved in the synthesis of biologically active molecules including:• ;Nitrotriazole amines or nitroimidazole amines for use as antitrypanosomal activity and mammalian cytotoxicity1• ;Quinazoline-containing piperazinylpyrimidine derivatives with antitumor activity2• ;Quinazoline substituted cyclopentane as HCV NS3/4A protease inhibitors3• ;Quinazolines with antibacterial and antitumor activity4• ;Aurora inhibitor MK-04575Reactant involved in Suzuki-Miyaura cross-coupling6 and catalyst-free / base-free water promoted nucleophilic aromatic substitution7
Safety Statements:37/39-26
37/39:Wear suitable protective clothing, gloves and eye/face
protection
26:In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice |
| Flash Point: | 164.4°C |
| Usage: | Reactnat involved in the synthesis of biologically active molecules including:• ;Nitrotriazole amines or nitroimidazole amines for use as antitrypanosomal activity and mammalian cytotoxicity1• ;Quinazoline-containing piperazinylpyrimidine derivatives with antitumor activity2• ;Quinazoline substituted cyclopentane as HCV NS3/4A protease inhibitors3• ;Quinazolines with antibacterial and antitumor activity4• ;Aurora inhibitor MK-04575Reactant involved in Suzuki-Miyaura cross-coupling6 and catalyst-free / base-free water promoted nucleophilic aromatic substitution7 |
| Safety Data |
| |
 |