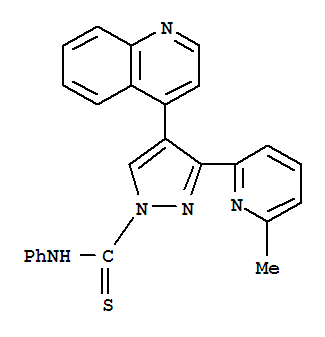
| Identification |
| Name: | 1H-Pyrazole-1-carbothioamide,3-(6-methyl-2-pyridinyl)-N-phenyl-4-(4-quinolinyl)- |
| Synonyms: | A 83-01; |
| CAS: | 909910-43-6 |
| Molecular Formula: | C25H19N5S |
| Molecular Weight: | 0 |
| InChI: | InChI=1/C25H19N5S/c1-17-8-7-13-23(27-17)24-21(19-14-15-26-22-12-6-5-11-20(19)22)16-30(29-24)25(31)28-18-9-3-2-4-10-18/h2-16H,1H3,(H,28,31) |
| Molecular Structure: |
 |
| Properties |
| Flash Point: | 310.6°C |
| Boiling Point: | 590°C at 760 mmHg |
| Density: | 1.27g/cm3 |
| Refractive index: | 1.706 |
| Biological Activity: | Selective inhibitor of TGF- β type I receptor ALK5 kinase, type I activin/nodal receptor ALK4 and type I nodal receptor ALK7 (IC 50 values are 12, 45 and 7.5 nM respectively). Blocks phosphorylation of Smad2 and inhibits TGF- β -induced epithelial-to-mesenchymal transition. Only weakly inhibits ALK-1, -2, -3, -6 and MAPK activity. More potent than SB 431542 (4-[4-(1,3-benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol -2-yl]benzamide). Inhibits differentiation of rat induced pluripotent stem cells (riPSCs) and increases clonal expansion efficiency. Helps maintain homogeneity and long-term in vitro self-renewal of human iPSCs. |
| Flash Point: | 310.6°C |
| Safety Data |
| |
 |