| Identification |
| Name: | Hydralazine hydrochloride |
| Synonyms: | 1(2H)-Phthalazinone hydrazone; 1-Hydrazinophthalazine hydrochloride |
| CAS: | 304-20-1 |
| EINECS: | 206-151-0 |
| Molecular Formula: | C8H8N4?HCl;C8H9ClN4 |
| Molecular Weight: | 160.17592 |
| InChI: | InChI=1S/C8H8N4/c9-11-8-7-4-2-1-3-6(7)5-10-12-8/h1-5H,9H2,(H,11,12) |
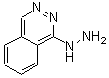
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 2811 6.1/PG 3 |
| Density: | g/cm3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility: | Soluble in water (50 mg/ml - clear, yellow to yellow-green solution), slightly soluble in ethanol; very slightly soluble in ether |
| Solubility: | Soluble in water (50 mg/ml - clear, yellow to yellow-green solution), slightly soluble in ethanol; very slightly soluble in ether |
| Appearance: | White Crystalline Solid |
| Specification: |
Hydralazine hydrochloride (304-20-1) is a direct-acting smooth muscle relaxant used to treat hypertension by acting as a vasodilator primarily in arteries and arterioles. By relaxing vascular smooth muscle, vasodilators act to decrease peripheral resistance, thereby lowering blood pressure.It is not used as a primary drug for treating hypertension because it elicits a reflex sympathetic stimulation of the heart (the baroreceptor reflex). The sympathetic stimulation may increase heart rate and cardiac output, and may cause angina pectoris or myocardial infarction.It may also increase plasma renin concentration, resulting in fluid retention.and it is used to treat severe hypertension, but again, it is not a first-line therapy for essential hypertension. However, hydralazine is the first-line therapy for hypertension in pregnancy, with methyldopa.but patients given hydralazine over a period of six months may develop a lupus-like syndrome or other immune-related diseases that, in general, are reversible with withdrawal.Hydralazine is differentially acetylated by fast and slow acetylator phenotypes, hence incidence of lupus-like disease in slow acetylators.
|
| Report: |
IARC Cancer Review: Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 , 1980,p. 85.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory.
|
| Packinggroup: | III |
| Color: | Yellow crystals
White, crystalline powder |
| Usage: | Inhibits DNA methyltransferase and modulates epigenetic regulation of gene expression. Non-selective MAO-A/B inhibitor; semicarbazide sensitive amine oxidase inhibitor. Antihypertensive |
| Safety Data |
| Hazard Symbols |
 Xn:Harmful
Xn:Harmful
|
| |
 |