| Identification |
| Name: | sec-butyl alcohol |
| Synonyms: | (Delta1)-2-Butanol; sec-Butyl alcohol 99.5+ % (GLC) for analysis; Butanol; 99%; (+/-)-2-Butanol; sec-Butyl alcohol |
| CAS: | 78-92-2 |
| EINECS: | 201-158-5 |
| Molecular Formula: | C4H10O |
| Molecular Weight: | 74.12 |
| InChI: | InChI=1/C4H10O/c1-3-4(2)5/h4-5H,3H2,1-2H3 |
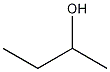
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 1120 |
| Density: | 0.8 |
| Stability: | Stable. Flammable. Substances to be avoided include acids, acid chlorides, acid anhydrides, oxidizing agents and halogens. |
| Refractive index: | 1.396-1.398 |
| Solubility: | Water Solubility :12.5 g/100 mL (20 oc) |
| Appearance: | Colourless liquid |
| Specification: |
?sec-Butanol (CAS NO.78-92-2) is also named as 1-Methyl propanol ; 1-Methyl-1-propanol ; 1-Methylpropyl alcohol ; 2-01-00-00400 (Beilstein Handbook Reference) ; 2-Butanol (natural) ; 2-Butyl alcohol ; 2-Hydroxybutane ; AI3-24189 ; Alcool butylique secondaire ; Alcool butylique secondaire [French] ; BRN 0773649 ; Butan-2-ol ; Butanol secondaire ; Butanol secondaire [French] ; Butyl alcohol, sec- ; Butylene hydrate ; CCS 301 ; Caswell No. 119C ;?Ethyl methyl carbinol ; Ethylmethyl carbinol ; HSDB 674 ; Methyl ethyl carbinol ; Methylethyl carbinol ; Methylethylcarbinol ; NSC 25499 ; s-Butanol ; s-Butyl alcohol ; sec-Butyl alcohol .?sec-Butanol (CAS NO.78-92-2) is colourless liquid with an alcohol odor. It is soluble in water. Moderately irritates the eyes and skin. Prolonged and repeated contact may cause defatting and drying of the skin. Vapors may irritate the nose, throat and respiratory tract. May be harmful by ingestion.?Acetyl bromide reacts violently with alcohols or water. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence.
|
| Report: |
Community Right-To-Know List. Reported in EPA TSCA Inventory.
|
| Packinggroup: | II |
| HS Code: | 29051490 |
| Storage Temperature: | Flammables area |
| Sensitive: | Hygroscopic |
| Color: | Colorless liquid |
| Usage: | Solvent. |
| Safety Data |
| Hazard Symbols |
 Xi:Irritant
Xi:Irritant
|
| |
 |