| Identification |
| Name: | 4-Methyl-3-nitrobenzoic acid |
| Synonyms: | 3-Nitro-p-toluic acid; 3-nitro-4-methyl benzoic acid; 4-Methyl -3-Nitrobenzoic acid; 4-methyl-3-nitro-benzoicaci; RARECHEM AL BO 1292; 3-NITRO-4-METHYLBENZOIC ACID; AKOS BBS-00007715; 2-NITROTOLUENE-4-CARBOXYLIC ACID; 4-Methyl-3-Nitrobenzoic Acid 3-Nitro-4-Methylbenzoic Acid; 4-Methyl-3-nitrobenzoicacid,99%; 4-Methyl-3-nitrobenzoic; 3-Nitro-p-toluylsure; 4-Methy-3-nitrobenzoic acid |
| CAS: | 96-98-0 |
| EINECS: | 202-549-3 |
| Molecular Formula: | C8H7NO4 |
| Molecular Weight: | 181.15 |
| InChI: | InChI=1/C8H7NO4/c1-5-2-3-6(8(10)11)4-7(5)9(12)13/h2-4H,1H3,(H,10,11) |
| Molecular Structure: |
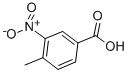 |
| Properties |
| Transport: | 50kgs |
| Density: | 1.392 g/cm3 |
| Stability: | Stable under normal temperatures and pressures. |
| Solubility: | Very soluble |
| Appearance: | white to light yellow crystal powder |
| Specification: |
?4-Methyl-3-nitrobenzoic acid (CAS NO.96-98-0) is also named as 3-Nitro-4-methylbenzoic acid ; 3-Nitro-para-toluic acid ; NSC 50659 ; m-Nitro-p-toluic acid ; 3-Nitro-p-toluic acid ; Benzoic acid, 4-methyl-3-nitro- ; p-Toluic acid, 3-nitro- (8CI) .?4-Methyl-3-nitrobenzoic acid (CAS NO.96-98-0) is white to light yellow crystal powder. It is?insoluble in water. 4-Methyl-3-nitrobenzoic acid is a nitrated carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry.?Flash point of 4-Methyl-3-nitrobenzoic acid is not available. It is probably combustible.
|
| Storage Temperature: | Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. |
| Safety Data |
| Hazard Symbols |
 Xn:Harmful
Xn:Harmful
|
| |
 |